To prevent medication errors, the U.S. Pharmacopeial Convention (USP) revised the label standard for two heparin products: Heparin Sodium Injection, USP and Heparin Lock Flush Solution, USP (including prefilled heparin flush syringes). As a result, the U.S. Food and Drug Administration (FDA) now requires manufacturers to change the labels of these products.
Instead of prominently listing the total content of the entire container, previous labels for these multidose vials showed the per-dose volume. To prevent dosage errors, the new labels display the container’s full volume, with the per-dose strength close by. You can learn more and view a side-by-side comparison of the labels in this FDA Drug Safety Communication.
Current Heparin Label

Revised Heparin Label
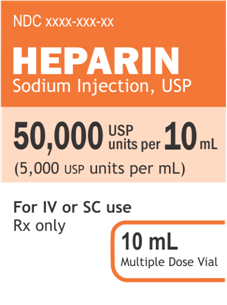
Label images courtesy of FDA
These changes took effect May 1, 2013, so you may already see the label changes in new stock. USP recommends the following steps to protect patients during this label transition:
- Ensure that key personnel are aware of and trained on these changes (pharmacists, nurses, physicians, leadership, buyers, risk management, informatics*).
- Consider separating old-label stock from new, and exhaust old supplies before introducing the new.
- Look at heparin vial labels before administering dosages to patients.
- Implement independent double-checks** for high-risk drugs, including heparin.
USP is a scientific nonprofit organization that sets standards for the identity, strength, quality, and purity of the medicines, food ingredients, and dietary supplements manufactured, distributed, and consumed worldwide. USP also sets standards for packaging and labeling; USP’s drug standards are enforceable in the United States by the FDA.
For more information on these label changes, USP offers a free, on-demand webinar for health care professionals and additional resources on its heparin labeling changes Web page.
*The label change will not affect bar codes/scanning. However, informatics staff may need to adjust computer systems because of the label change.
**USP member Institute for Safe Medication Practices (ISMP) offers more information on independent double-checks.